-

Molecular sab cib JZ-ZMS3
-

Molecular sab cib JZ-ZMS4
-

Molecular sab cib JZ-ZMS5
-

Molecular sab cib JZ-ZMS9
-

Molecular sab cib hmoov jz-zt
-

Molecular sab cib JZ-AZ
- Kev piav txog
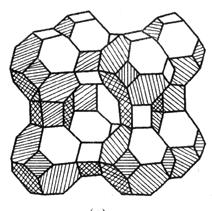
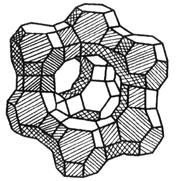
- Cov molecules ntawm cov tshuaj sib txawv yog qhov txawv ntawm qhov tseem ceeb thiab qhov loj ntawm kev kos duab, yog li cov duab hu ua "molecular sab cib".
- Molecular sieve (also known as synthetic zeolite) is a silicate microporous crystal. Nws yog cov pob txha pob txha uas yooj yim muaj cov khoom sib xyaw ua ke ntawm silicon aluminate, nrog rau cov hlau xaim (k +, thiab lwm yam) kom sib npaug cov kev tsis zoo ntawm cov siv lead ua. The type of molecular sieve is mainly divided into A type, X type and Y type according to its crystal structure.
|
| 2) x (sio.22O. |
|
|
|
|
| Lub cev pob txha ntawm cov Zeolite muaju, nrog cov duab sib txawv ntawm qhov thiab raws |
| H2O | Lub cev muaj kab mob lub cev tau kos duab |
| Nta | Ntau yam adsorption thiab kev cawm dim yuav ua tau |
|
| | Lub ntsiab tivthaiv ntawm hom molecular sab nrov yog silicon aluminate. Lub ntsiab crystal lub qhov yog lub psosaring.the aperture ntawm lub crystal aperture yog 4å (1Å Txauv ntawm CA2 + rau NA + nyob rau ntawm 4a molecular sab cib, sib sau ib lub aperture ntawm 3A, uas yog ib tug 3a (Oka Potassium a) cov roj ntsha sab. |
|
|
|
- Daim ntawv thov
- Cov adsorption ntawm cov khoom siv los ntawm kev kos npe rau lub cev (vander WATORS yuam), nrog lub qhov muaj zog polarbation thiab courtal molecules (xws li dej) thiab unsaturated molecules.
- Lub aperture faib ntawm cov suab paj nruag molecular yog cov khoom siv tsis zoo, thiab tsuas yog cov tshuaj nrog cov kab sib chaws me me dua lub qhov taub uas hla hauv lub qhov sab hauv ntawm cov nplais molecular.